FLEXITOUCH® PLUS
Clinically Proven Lymphedema Relief
For a better long-term outcome, help head and neck cancer patients address lymphedema symptoms from the convenience of their homes.
NEWLY PUBLISHED RCT DATA FROM VANDERBILT
Flexitouch shows improvements in:
External Swelling
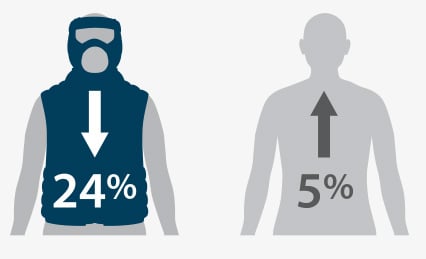
(p <0.001)*
*Statistically Significant Results
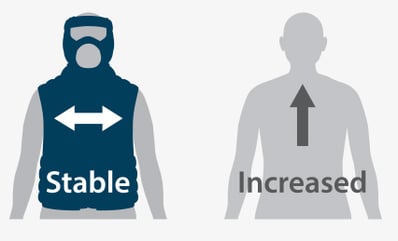
Swallowing Solids

(p=.016)*
*Statistically Significant Results
Level of Pain
(p=0.008)*
*Statistically Significant Results
Flexitouch Group:
n = 19
Self-Management Group
n = 24
Flexitouch also showed:
Improvement in
- Ability to control lymphedema at home (p=0.003_
- Mucous related symptoms (p=0.050)
- Severity of soft tissue symptoms (p=0.008)
- Neurological symptoms (p=0.047)
Decreases in face swelling
- Front view (p<0.001)
- Left view (p=0.005)
- Right view (p=0.004)
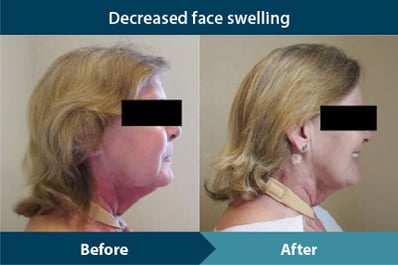
Results are based on the median change in scores from baseline measurements
Ridner, S.H., Dietrich, M.S., Deng, J. et al. Advanced pneumatic compression for treatment of lymphedema of the head and neck: a randomized wait-list controlled trial. Support Care Cancer (2020). doi.org/10.1007 /s00520-020-05540-8
Ridner, S.H., Dietrich, M.S., Deng, J. et al. Advanced pneumatic compression for treatment of lymphedema of the head and neck: a randomized wait-list controlled trial. Support Care Cancer (2020). doi.org/10.1007 /s00520-020-05540-8
Read additional clinical studies
 Vanderbilt University Study
Vanderbilt University Study
Supportive Care in Cancer, 2020
First-ever randomized control trial of home management of head and neck lymphedema
 Longitudinal Patient Reported Outcomes Study
Longitudinal Patient Reported Outcomes Study
Head and Neck Journal 2020
Retrospective analysis of 205 Flexitouch user survey responses
 Flexitouch Impact on Lymphatic Movement
Flexitouch Impact on Lymphatic Movement
Otolaryngology - Head and Neck Surgery 2019
Near-infrared fluorescence lymphatic imaging (NIRFLI) before and after Flexitouch use
Flexitouch Plus is the only FDA cleared pneumatic
compression device for head and neck cancer-related lymphedema and can be prescribed
during routine follow up care.
It takes just 32 minutes per day.
See the clinical benefit of Flexitouch Plus
Swelling six week post cancer treatment
Late-effect fibrosis seven years post cancer treatment



